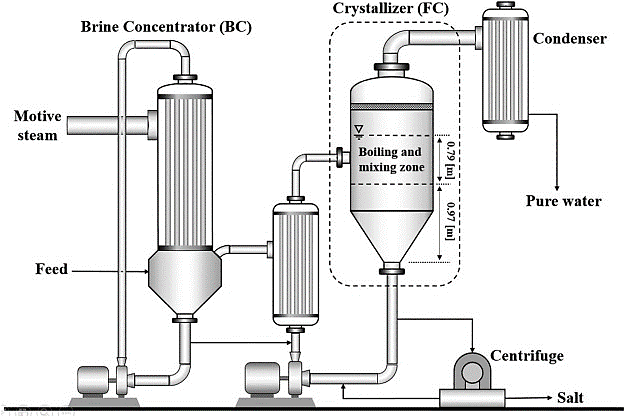
Crystallization is a physical change. Crystallization is the formation of solids from the liquid or gaseous phase. This technique includes obtaining the crystals of a soluble substance from a hot saturated solution and separating the soluble solid from the solution.
To concentrate feed into solid crystals and clean water, crystallizers are used. A progressively harder method for which crystallite sizes are formed from a liquid solution is known as crystallization. Crystallizers can remove liquid wastes completely, resulting in no liquid discharge (ZLD). The process continues and secondary crystallization are the two stages of crystallization. The formation of new crystals is referred to as primary nucleation. Secondary nucleation is the primary stage that results in the mass production of crystals. There are two types of crystallization processes: evaporative crystallization and cooling crystallization.
Evaporative crystals are used in the whole process of evaporative crystallization. As a result of this crystallizer, the solvent evaporates bringing about an increase in the concentration of the answer.
Salt crystallization inhibitors are recognized to get the potential of efficiently controlling the morphology of precipitating salts even at a very small total.
For crystal advancement, a zone is when crystals come into connection with the supersaturated solution. In certain conditions, crystals are suspended throughout the vessel by agitation; though in Many others, crystals occupy only a percentage of the vessel, normally for a fluidized mattress.
Sources which include analysis and screening facilities, in-dwelling multi-disciplined engineering groups, and a single-stage task management design make it possible for a comprehensive overview in the undertaking to the client. This flexible approach helps make Veolia a favored spouse for FEED or phased engineering contracts for highly elaborate projects.
The large pore quantity with the crust layer can make the obtainable MgSO4·7H2O unable to entirely fill the pore Place One of the NaCl crystals, always protecting enough pore space obtainable for water transport towards the evaporation area.
Within the 3D crystallizer, warmth is done from the aluminum sheet surface area towards the inner aspect 1st after which you can further more to your outer area from the QGF membrane. Furthermore, drinking water evaporation is endothermic and an interfacial procedure, which will take spot only at the outer surface area on the QGF membrane. As a result, the outer area from the QGF membrane possesses a lessen temperature and better salt concentration as opposed to internal aspect of a similar membrane, bringing about a selective precipitation of NaCl salt crystals only over the outer surface38.
Exceptional photothermal conversion efficiency can boost h2o evaporation and salt crystallization. The solar absorption of the crystallizer at the total-spectrum wavelength is proven in Figure 6a, reaching 93%. It indicated which the photothermal layer of your crystallizer experienced fantastic light-absorption capacity. As shown in Figure 6b, an indoor photo voltaic simulation system was applied To guage the drinking water evaporation effectiveness. As revealed in Determine 6c, the h2o evaporation amount with the crystallizer amplified as the angle between the photothermal levels greater. This is due to the height in the crystallizer itself turning into smaller sized as being the angle among the photothermal layers gets to be larger, after which you can the brine was able to get to the photothermal layer far more rapidly. Once the crystallizer were working for a specified interval, the reliable salt was gathered from the crystallizer surface in addition to within the buoyancy layer. The reliable salt gathered was dried at fifty five °C for 6 h to a continuing excess weight. As revealed in Figure 6c, d, the angle involving the photothermal layers performed a crucial job from the evaporating amount in the crystallizer.
Our SaltMaker® household of providers provides scalable, adaptable solutions that address all evaporation and crystallization demands, such as Restoration of useful metals, and MLD and ZLD.
The aggressive advantage of the outer surface in salt precipitation retains the internal side of your membrane free of salt crystals (Fig. 3d) and maintains the water channel In the QGF membrane unobstructed.
Veolia could be the market leader in industrial crystallizers which generate chemical substances including ammonium sulfate
Disposal of stable waste in landfills can leach substances into groundwater and may cause groundwater pollution.
The administration of hypersaline brines (which is, wastewater of higher salinity) is really a technological challenge which includes obtained rising focus because of their escalating volume, environmental impacts and increasingly stringent restrictions. Right here we existing electrodialytic crystallization (EDC) as a fresh system to accomplish brine crystallization without having evaporation. Within an EDC procedure, the brine stream recirculating concerning Crystallizer Manufacturer Crystallizer For Zero Liquid Discharge System an electrodialysis cell plus a crystallizer continues to be oversaturated by way of ongoing electromigration of ions in the feed stream over the ion Trade membranes. We first utilised Na2SO4 given that the product salt to show the feasibility of EDC and to conduct a systematic investigation of how crystallization kinetics and crystal size distribution rely on latest density and crystallization manner.
It needs to be cautiously identified which the solar crystallizer at this point may not be quite powerful in treating large quantity of your brine as a consequence of its significant land prerequisite. Nonetheless, evaluating Along with the industrial ZLD system, which costs $250000 to around $2 million with the ability of 5–one hundred m3 each day (), the photo voltaic crystallizer offers a very low-barrier-of-entry option for managing brine with little to medium quantity. For instance, for just a medium-sized chemical plant generating around 15 m3 concentrated brine everyday, there will be a location of around 300 m2 photo voltaic crystallizer array plus interdevice Room needed to deal with the brine with ZLD.
So, the NaCl salt crust tends to progress alongside the out-of-airplane direction to slowly raise its thickness and to maintain its porosity comparatively reliable. The porosity of the salt crust formed by pure NaCl brine (20 wt%) is 19.3% depending on mercury intrusion porosimetry measurement. As illustrated in Fig. 5a, the loosely packed pure NaCl salt crust layer permits liquid drinking water and drinking water vapor transportation, which clarifies the secure water evaporation efficiency with pure NaCl brine.